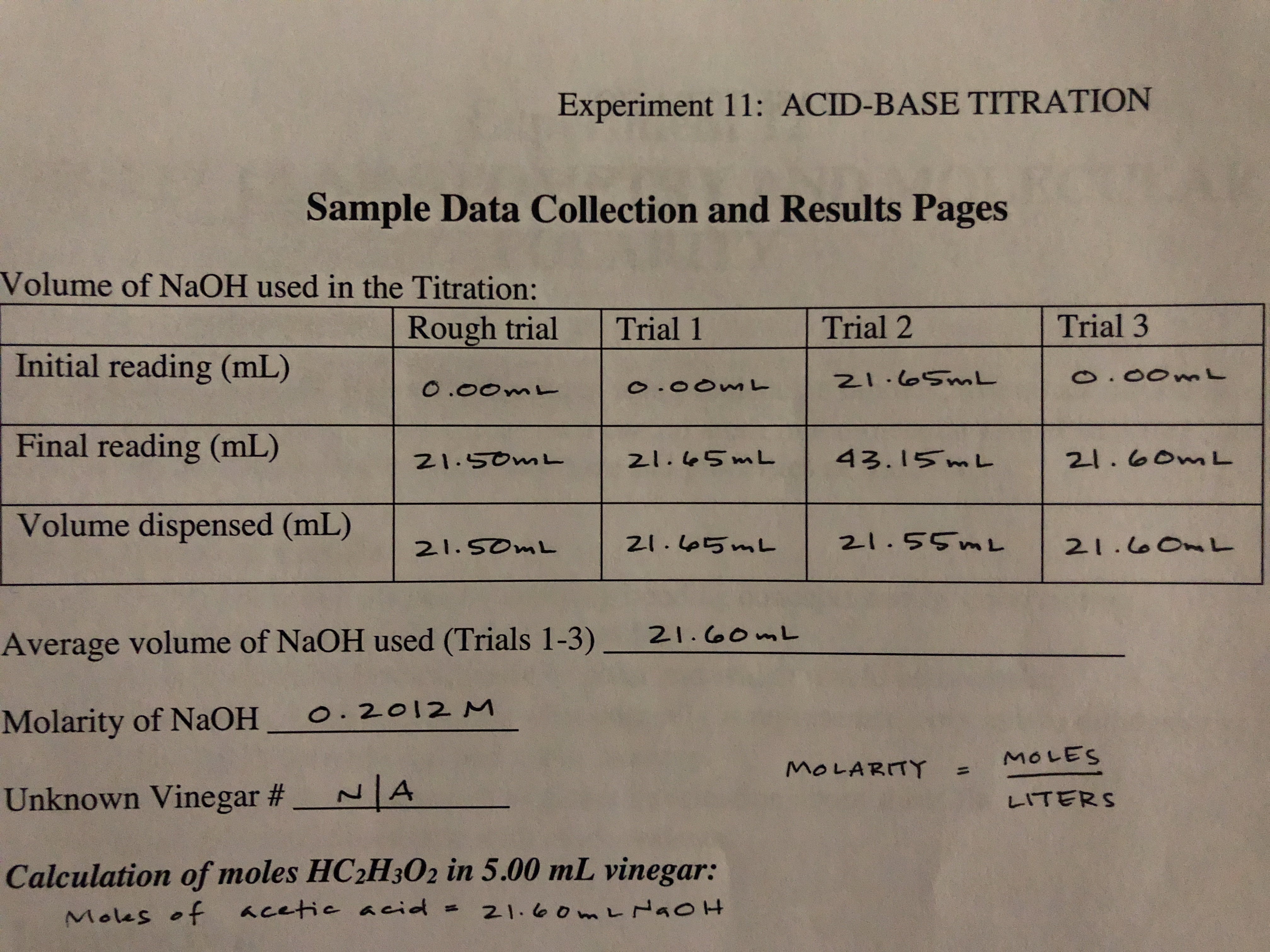
Vinegar is a mixture of acetic acid and water. This experiment is designed to determine the molar concentration of acetic acid in a sample of vinegar by titrating it with a standard solution of NaOH.

From the balanced chemical equation.
Titration of vinegar post lab answers. Titration of Vinegar Post Lab Questions. Briefly explain why adding laboratory water to the vinegar sample in the reaction flask prior to beginning the titration does not affect the results of your determination of the molarity of CH3COOH in your vinegar sample. The vinegar in this titration was distilled vinegar and is clear and colorless.
Chemistry questions and answers. Titration for Acetic Acid in Vinegar-Lab Report Exercise 1. Determining the Concentration of Acetic Acid Data Table 1.
NaOH Titration Volume Initial NaOH Volume mL 859 920 920 Final NaOH Volume Trial 1 Trial 2 Trial 3 mL 020 100 201 Total volume of NaOH used mL 839 820 719 Average Volume of NaOH Used. Vinegar is a solution of acetic acid CH 3COOH or HC 2H 3O 2 in water. The amount of acetic acid is usually 5 by mass in the vinegar solution.
In this experiment you will determine the mass percent of acetic acid in vinegar by titration. Titration is a common method used by chemists to find the concentration of a substance in a solution. Vinegar is a solution of acetic acid CH 3 COOH or HC 2 H 3 O 2 in water.
The amount of acetic acid is usually 5 by mass in the vinegar solution. In this experiment you will determine the mass percent of acetic acid in vinegar by titration. Titration is a common method used by chemists to find the concentration of a substance in a solution.
In this experiment we will perform a titration a lab technique that is used to determine the concentration of a solute in a solution. We will use titration to find the molar concentration and massmass percent of acetic acid in vinegar. During the titrations in this experiment we will neutralize an acid solution by slowly adding NaOH solution using a buret.
A buret is a tool used to. Vinegar Analysis Formal Report Introduction. Formal Laboratory ReportC125 Experimental Chemistry ISection 20500IUPUINovember 09 2018Finding Unknown Concentration using TitrationINTRODUCTIONMost people have faced some kind of stomach problem in their lives.
One of themost common stomach problem is having too much stomach acid. Titrating the vinegar sample. Use the blue ____ _____ with the _____ ____ to draw 5 mL of _____ sample into the pipet.
Remove the _____ and stop the flow with your index finger. _____ the pipet to an almost. This technique increases the reported acetic acid in the vinegar because the person should have waited the 10-15 seconds before recording the initial volume of the buret.
If the person did this they would have accurately found the difference between the. Post Lab Assignment Acid-Base Titration CHEM 131L Name. Gabrielle Miner Lab Section Number.
Acid Base Titration Due Date. 61416 DATA CALCULATIONS. Amounts of NaOH and Molarity of Acetic Acid Trial Number Initial Volume NaOH mL Final Volume NaOH mL Total NaOH mL moles of NaOH Molarity of acetic acid.
Data Table P1. Titration of acetic acid with calcium hydroxide concentration of CaOH 2 0361 M volume vinegar solution 1200 mL mass vinegar solution 1206 g volume of CaOH 2 solution 1152 mL mmol of CaOH 2 416 mmol mmol of HC 2 H 3 O 2 832 mmol mass of HC 2 H 3 O 2 0500 g mass of HC 2 H 3 O 2 in the original sample 415 molarity of HC 2 H 3 O 2 in the original sample 0693 M Additional Materials Titrations Periodic Table Lab 9 PostLab - Titrations. Open 18 pages titration of vinegar lab report answers explanation in PDF format.
In this lab you will perform a titration using sodium hydroxide and acetic acid in vinegar. Titration for acetic acid in vinegar lab report Solved. Then add about 20-mL of distilled water and 2-3 drops of phenolphthalein to this Erlenmeyer flask.
Explain the difference between the two cases. Within each flask we added 20-30 mL of deionized water into the 5mL of vinegar and 5 drops of phenolphthalein indicator solution. Since we mixed vinegar and water in a flask there was not a need to dry the flask because just water was being added.
Titration of Vinegar prepare these samples in triplicate Step 8. Pour approximately 090 mL of vinegar into the clean dry graduated cylinder from step 2. Using the berel pipet with the label a add more vinegar solution dropwise so the volume is precisely 100 mL.
Pour this solution into a clean test tube. Obtain 15-20 mL of vinegar in a clean and dry 100 mL beaker. Record the brand name of the vinegar.
Using a 10-mL volumetric pipette carefully pipet 10 mL of vinegar into a 100-mL volumetric flask. Fill the flask to the 100 mL mark using distilled or deionized water. Use this diluted vinegar to perform the titrations.
How did the value for the percent acetic acid in vinegar that you determined compare with that claimed by the manufacturer. Calculate the error. Comment on how you could improve your result.
While carrying out a titration explain why is it. Chemistry questions and answers. LAB 15 Analysis of Vinegar INTRODUCTION The purpose of this experiment is to perform an acid-base titration and calculate the concentration of acetic acid in a sample of vinegar.
Background Information Chemists perform titrations in the laboratory to precisely determine solution concentrations. The most common type of titration is the acid-base titration. In this experiment you will determine the concentration of acetic acid HC 2 H 3 O 2 in commercial vinegar.
Vinegar is a mixture of acetic acid and water. In this titration aqueous NaOH is the titrant and vinegar is the analyte. Each person must perform a titration.
For those working in groups each group member will contribute the results of one determination to the group effort. Your reported result will be the average of at least two titrations. Sample Calculation Calculating the concentration M of CH 3 COOH in commercial vinegar.
From the balanced chemical equation. I The burette acquired from the lab cupboard was not rinsed before students filled it with the standard base solution. Ii The Erlenmeyer flask or beaker placed below the burette had been rinsed thoroughly with water after completing the first titration and was reused for the second titration rather than acquiring a new clean dry beaker.
A 500 mt sample of vinegar has a concentration of 0800 M. What volume of 0150 M NaOH is required to complete the titration. A 100 ml sample of household ammonia NH3aq is titrated with 05 M HCI.
If 257 ml of acid is required what is the concentration of. According to the theory of acid-base titrations the end point in the titration of vinegar with sodium hydroxide will be observed between pH 8 and 10. Therefore in this titration also phenolphthalein is a siutable indicator.
The reaction between vinegar and sodium hydroxide is given by CH 3 - COOH NaOH CH 3 COONa H 2 O. Titration of Vinegar Lab Answers. Vinegar is a common household item containing acetic acid as well as some other chemicals.
This experiment is designed to determine the molar concentration of acetic acid in a sample of vinegar by titrating it with a standard solution of NaOH.