For preparation of Aspirin acetic anhydride is added to the measured amount of salicylic acid. The weight of the recovered sample was 285g.
The weight of the recovered sample was 285g.
Synthesis of aspirin lab report answers. Chemistry questions and answers. Aspirin Synthesis - Lab Report Questions. Describe one safety practice important to this lab experiment.
Explain you answer specific to the chemistry and procedures used in this lab. 2Sometimes when you open an old bottle of aspirin you can detect a vinegar-like smell. For preparation of Aspirin acetic anhydride is added to the measured amount of salicylic acid.
Sulphuric acid is added and heated for a short period to complete reaction. Water is added once removed from heat with addition of cold water and suction filtration is carried out. As for recrystallisation of aspirin collected crude product prepared in preparation of aspirin which is impure is dissolved in ethanol and hot.
Synthesis of Aspirin Conclusion In Part 1 the purpose of the lab is to prepare acetylsalicylic acid commonly called aspirin and determine the experiment yield of a synthesis of Aspirin. Lab report answers for aspirin synthesis Yahoo Answers. The weight of the recovered sample was 285g.
Through your references I answered my own papers. Obtain a capillary tube from your instructor and gently press the open end into the pile of aspirin crystals on the paper so that a few crystals of aspirin enter the capillary tube. Tap the closed end of the capillary onto the bench top so that the aspirin crystals work their way to the bottom.
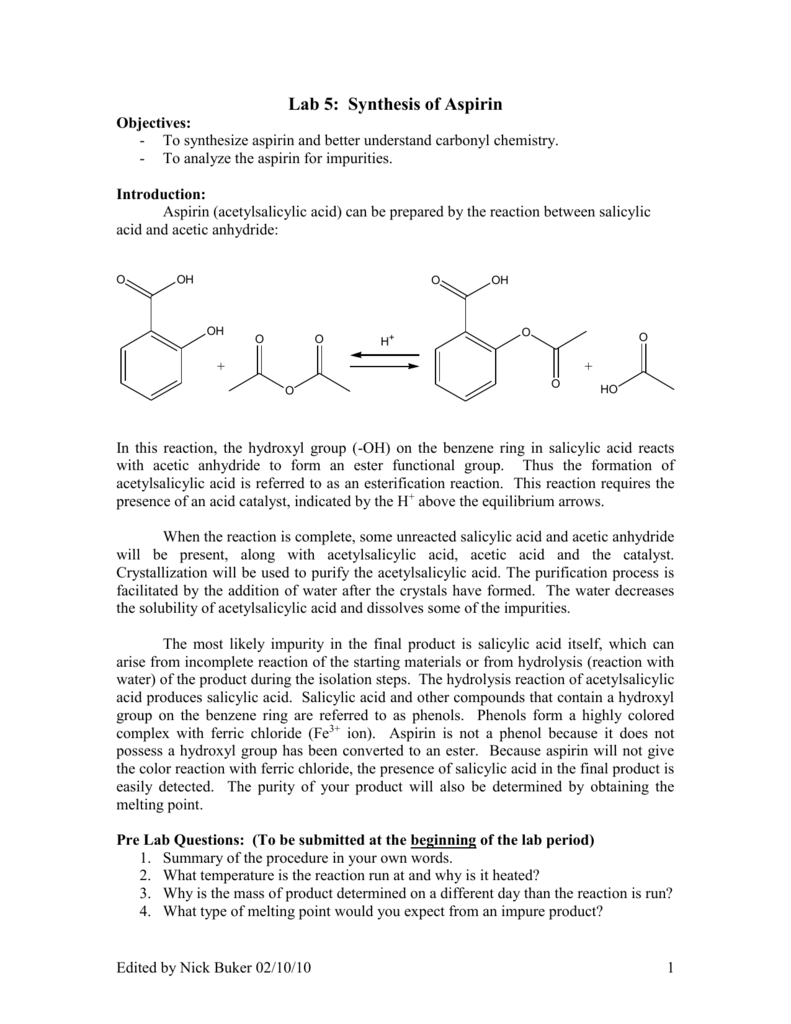
Synthesis of Aspirin Aspirin is the single most manufactured drug in the world. Aspirins chemical name is acetylsalicylic acid and it is synthesized from the reaction of acetic anhydride with salicylic acid in the presence of phos-phoric acid as a catalyst. The by-product is acetic acid Figure 51.
Aspirin Synthesis and Analysis Revised. 121314 Crush an aspirin tablet and place in a labeled pre-weighed vial or test tube. Record the mass of the aspirin tablet.
Do not lose this container or its contents you will use them later. Take a small amount of the aspirin tablet and dissolve as much as you can in 3 drops of ethyl acetate. 1 mol aspirin 18017 g aspirin 00305 mols salicylic acid 550 g aspirin 1 mol salicylic acid 1 mol aspirin 392 g Yield 100 713 550 g.
Synthesis of Aspirin Lab Report. The goal of this experiment was to synthesize aspirin. In this experiment aspirin also known as acetylsalicylic acid was synthesized from salicylic acid and acetic anhydride.
In the reaction the hydroxyl group on the benzene ring in salicylic acid reacted with acetic anhydride to form an ester functional group. The Synthesis of Aspirin Chemistry Standard Level Lab Report Data Collection and Processing and Conclusion and Evaluation Date. December 8th 2011 Purpose.
The purpose of this lab was to synthesize aspirin determine the theoretical yield compare the percent yield to the theoretical yield and test the purity of aspirin by adding Iron III chloride to the product. During the preparing aspirin lab activity a student attempts to determine how much aspirin she made. She looked back at her data.
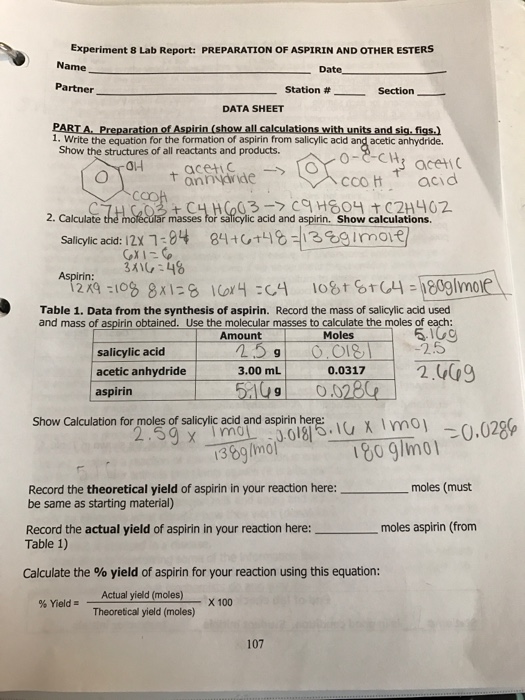
Mass of flask 7386 g _ mass of flask and salicylic acid 7788 g __ mass of salicylic acid 402 g mass of watch glass filter paper 3463 g. 240138 0017391 mol mol wt salicylic acid 138 Expected number of moles of aspirin f 0010507 mol Expected mass of aspirin g 001739 x 180 31302 g mol wt 180 percent d g - 100 463 melting point temperature range 1342 C to 1361 C Appearance White thin scaly crystals discussion My results After my experiment and. Chemistry questions and answers.
Lab Report Chapter 26 A. Preparation of Aspirin 1. Mass of flask 13674 2.
Mass of flask and salicylic acid 13874 3. Mass of watch glass 3674 4. Mass of watch glass and Aspirin product 3893 Calculations 5.
Mass of salicylic acid —– 6. Maximum theoretical yield of aspirin Show calculation. Primarily used to treat pain as well as to avert cardiovascular disease.
The purpose of this lab is to synthesize and characterize high purity aspirin. The aspirin synthesized in this experiment will be crystallized from solution. Crystallization will be induced by scratching the glass beaker to create nuclei around which aspirin may crystallize.
Procedure - Synthesis of Aspirin. Review calculations on pages 9 and 10 of lab notebook. Obtain mass of dry salicylic acid.
Do reaction using 90 of that salicylic acid. Calculate amount of acetic anhydride needed amount of salicylic acid times 3 2. Transfer 90 of salicylic acid into a 50 mL Erlenmeyer flask.
Start studying Experiment 8 Synthesis of Aspirin. Learn vocabulary terms and more with flashcards games and other study tools. Aspirin Synthesis Lab Part 1.
The Synthesis of Aspirin Procedure Steps 3 - 5 were repeated twice more for two more titrations. Preparation and Standardization of HCl Procedure Part 4. Quantitative Analysis of Aspirin Further Analysis Conclusion The overall objectives of.
Aspirin is a pain relieving compound that most students will be familiar with thus its synthesis gives students an insight into how chemistry is used in real-life applications. The synthesis of aspirin may be achieved in one simple step O-acetylation of salicylic acid Figure 1 which is incorporated into many undergraduate synthetic chemistry. Using the theoretical and actual you can calculate percent yield.
Actual Yield of Aspirin in lab after filtereddried mock value 370 g. Mass of Theoretical Yield of Aspirin mock value 3603 g. Percent Yield Actual Theoretical x 100 370 3603 x 100 103.
Your value may vary from the theoretical value for a number of reasons. Experiment 8 Synthesis of Aspirin Aspirin is an effective analgesic pain reliever antipyretic fever reducer and anti-inflammatory agent and is one of the most widely used non-prescription drugs. The use of aspirin had its origin in the 18th century when it was found that an extract from the bark of willow trees was useful in reducing.
The synthesis of aspirin is an organic chemistry experiment in many specifications for students of ages 16-18 years. Each of the four levels take approximately 30 minutes to complete and are designed to be used as pre-lab activities in class or as homework. Rasan Cherala Synthesis of Aspirin lab Purpose.
The purpose of this experiment is to create aspirin by reacting salicylic acid with acetic anhydride. This product will be impure because of unreacted salicylic acid and visible spectroscopy can help.