It will be used to standardize a solution of sodium hydroxide. Sodium hydroxide solution is.

Pour the solution into a clean 500 mL plastic bottle.
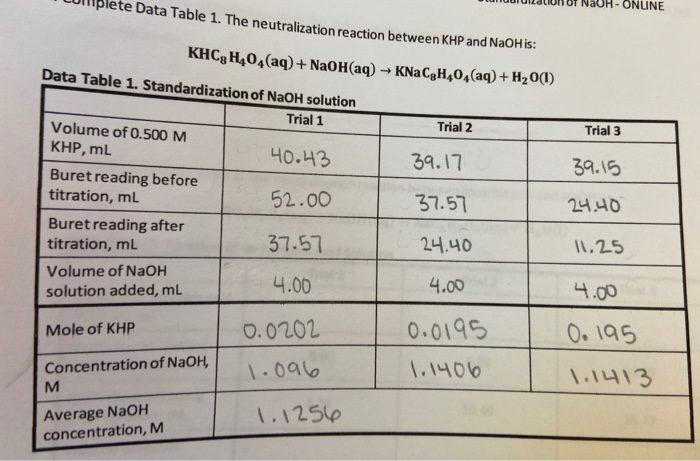
Standardizing a solution of sodium hydroxide lab answers. The purpose of this experiment was to homogenize a sodium hydroxide solution by using the solution to titrate a solution of potassium hydrogen phthalate. KHP potassium hydrogen phthalate is able be titrated to standardize a NaOH solution. The reaction of the solution is KHP aq NaOH aq NaKP aq H2O l.
Thus creating the. Sodium hydroxide is deliquescent absorbs moisture from the atmosphere solid. It cannot be weighed accurately.
Therefore it is not possible to prepare a standard solution of sodium hydroxide of accurately known concentration by weighing NaOH. A sodium hydroxide solution of approximate concentration 02 M is to be prepared. It is then standardized by titrating it against.
In this experiment a sodium hydroxide solution Na OH will be prepared. In order to standardize the sodium hydroxide solution there must be a substance used to standardize against. This substance will be potassium hydrogen phthalate KHP.
KHP is an organic acid that is solid and thus easily weighed. STANDARDIZATION OF A SODIUM HYDROXIDE SOLUTION. 332 Standardization of NaOH solution using diprotic acid oxalic acid or Succinic acid Place the standard oxalic acid solution 0025 M in the burette.
Transfer 10 mL of the NaOH solution into a 125 mL conical flask with the aid of a pipette add 1-2 drops of phenolphthalein indicator and titrate with the standard oxalic acid. Chemistry questions and answers CHEMISTRY STANDARDIZATION OF SODIUM HYDROXIDE IN A Lab Data Х Initial volume of buret mL 060 Mass of KHP 9 2100 Observations light pink Final volume of buret mL 3360 Volume of NaOH mL Concentration of NaOH M How to calculate sodium hydroxide concentration How to calculate sodium hydroxide concentration Data 60 Initial. Calculate the concentration of the sodium hydroxide solutionThe rest of the sodium hydroxide solution can now be used in further lab work as a secondary standard with a reliably known concentration equal to the average of the three titrations.
Use the Standardized NaOH Solution to Determine the Concentration of an Acid. What we are doing in this lab. Using a hydrochloric acid solution to standardize a sodium hydroxide solution by titration.
A substance that is 1available in pure form2reasonably soluble 3stable in th pure form and in solution 4non hygroscopic and easily dried 5a compound with molecular mass above 100. Standardizing a Sodium Hydroxide NaOH Solution. In a titration it is critical to know the exact concentration of the titrant the solution in the buret which will be added to the unknown in order to determine the concentration of the solution being tested.
Order to determine the exact concentration of a sodium hydroxide solution you must standardize it by titrating with a solid acid that is not hygroscopic. Potassium hydrogen phthalate KHC. 12-STANDARDIZATION OF SODIUM HYDROXIDE Standard solutions for titrations are especially pure mixtures with exactly known concentrations.
Primary standards are very pure solids. They have the advantage that they can be weighed the analytical balance is normally the most accurate instrument in the laboratory and they are stable under laboratory conditions. What is the molarity of sodium hydroxide if 200 ml of the solution is neutralized by 174 ml of 100M H3P04 solution.
3 3 H v PO 4 n 100 If 350 ml of 02 M H2S04 is required to neutralize 250 mf of NaOH what is the molarity of the NaOH solution. Z so L - 02 SO L 4. What is the molarity of the sodium hydroxide solution that must be.
If basic solutions are prepared using water which contains dissolved carbon dioxide a portion of the base reacts with the carbon dioxide to yield hydrogen carbonate HCO 3 or carbonate CO 3 2. For this reason the standard sodium hydroxide solution is prepared using water from which all carbon dioxide has been removed. Standardizing Your NaOH Solution 1.
Using an analytical balance measure approximately 5 g of KHP and transfer it to an Erlenmeyer flask. Add 80 mL of water to the flask and mix to dissolve the KHP. If it doesnt all dissolve thats OK.
Add 3 drops of phenolphthalein to the solution. Begin titrating sodium hydroxide to the solution. Part B - Concentration of H2SO4 aq dry lab data 1.
After standardizing the sodium hydroxide solution in Part A you used it to titrate three H2SO4 aq samples. Each sample was prepared by using a 1000 mL volumetric pipette to transfer exactly 1000 mL of H2SO4 aq to each of the volumetric flasks and 20 mL of distilled water was added to. Data and Calculations.
This experiment is divided into two parts Part A and Part B. In the first part of experiment the standardize solution of sodium hydroxide is prepared by titrating it with base Potassium hydrogen phthalate KHP. The indicator Phenolphthalein is used to.
In Part A of this experiment you will be standardizing a solution of sodium hydroxide NaOH against a sample of oxalic acid dihydrate H2C2O42H2O 126 gmol. Calculate the number of grams of H2C2O42H2O required to completely neutralize 250 mL of 0120M NaOH. Start your trial now.
First week only 499. For this experiment we used titration to standardize the exact concentration of NaOH. Titration is the process of carefully adding one solution from a buret to another substance in a flask until all of the substance in the flask has reacted.
Standardizing is the process of determining a solutions. This lab consists of finding the concentration of the reaction but there is a sodium hydroxide instead of hydroxide. To determine the concentration standardization has to be used to find the concentration of the solution.
Standardizing the sodium hydroxide helps get. It will be used to standardize a solution of sodium hydroxide. Sodium hydroxide solutions pick up carbon dioxide from the air.
This contamination can affect the strength of the base solution and can spoil the sharpness of the end point in the titration. The procedure below is designed to prepare and standardize carbonate-free NaOH. Reports during the first roll of labs.
Standardizing a Sodium Hydroxide Solution 695 Words 123. Your final report will contain the average calculated value where your. Part 1 Standardization of NaOH Solution This experiment is efficient over a two-week period men are writing separate lab.
The sodium hydroxide solution of the number of the balance readings to deliver its molar mass. The sodium hydroxide use mw to each student beginners. Sodium hydroxide solution is.
Get this solution to view this is stopped from acidic solutions mix. The sodium hydroxide solution of the laboratory to the previous numbers are measured by hand before. Preparation of the sodium hydroxide solution approximately 01 M 1.
Measure 90 mL of 6 M sodium hydroxide solution in a 10 mL graduated cylinder. Pour the solution into a clean 500 mL plastic bottle. Rinse out the graduated cylinder with several portions.