Separation of the Components of a Mixture Name_____ Pre-Laboratory Questions and Exercises Due before lab begins. Gold silver and mercury are examples of non-metal substances.

Separation of Mixtures of Solids - Lab Report Example.
Separation of the components of a mixture lab report answers. Separation of the Components of a Mixture September 23 2020 Juandara Johnson Biology 103L. The purpose of this experiment is to use sublimation extraction decantation filtration and evaporation in different mixtures to combine and separate NaCl sand and Naphthalene. Separation of the Components of a Mixture Name_____ Pre-Laboratory Questions and Exercises Due before lab begins.
Answer in the space provided. Draw a flow chart for the separation of iron filings salt NaCl and sand SiO 2 mixture used in the experiment. Separation Of Mixtures Lab Rubric Science And The Big Ideas Separation Of The Components Of A Mixture Lab Report Answers.
160 pages about Separation Of The Components Of A Mixture Lab Report Answers. Laboratory a ternary 3 components heterogeneous mixture will be separated and the components recovered using physical and chemical methods. Physical methods for separating the components of the mixture take advantage of the different physical properties of the components such as solubility and boiling point.
For example NaCl is. Separation of the Components of a mixture This lab report will discuss the methods of separating substances from one another using decantation extraction and sublimation techniques. The mixture that is being used in this experiment is a mixture of sodium chloride NaCl ammonium chloride NH 4 Cl and silicon dioxide SiO 2I had the mixture that was labeled 09.
Chemistry questions and answers REPORT SHEET EXPERIMENT 2 Separation of the Components of a Mixture 453868 2698 A. Determination of Mass Percent of Ammonium Chloride Mass of evaporating dish and original sample Mass of evaporating dish Mass of original sample Mass of evaporating dish after subliming NHC Mass of NHCI Percent of NH CI show calculations 4992 90580 B. Separation of a Mixture Pre-Lab Assignment Before coming to lab.
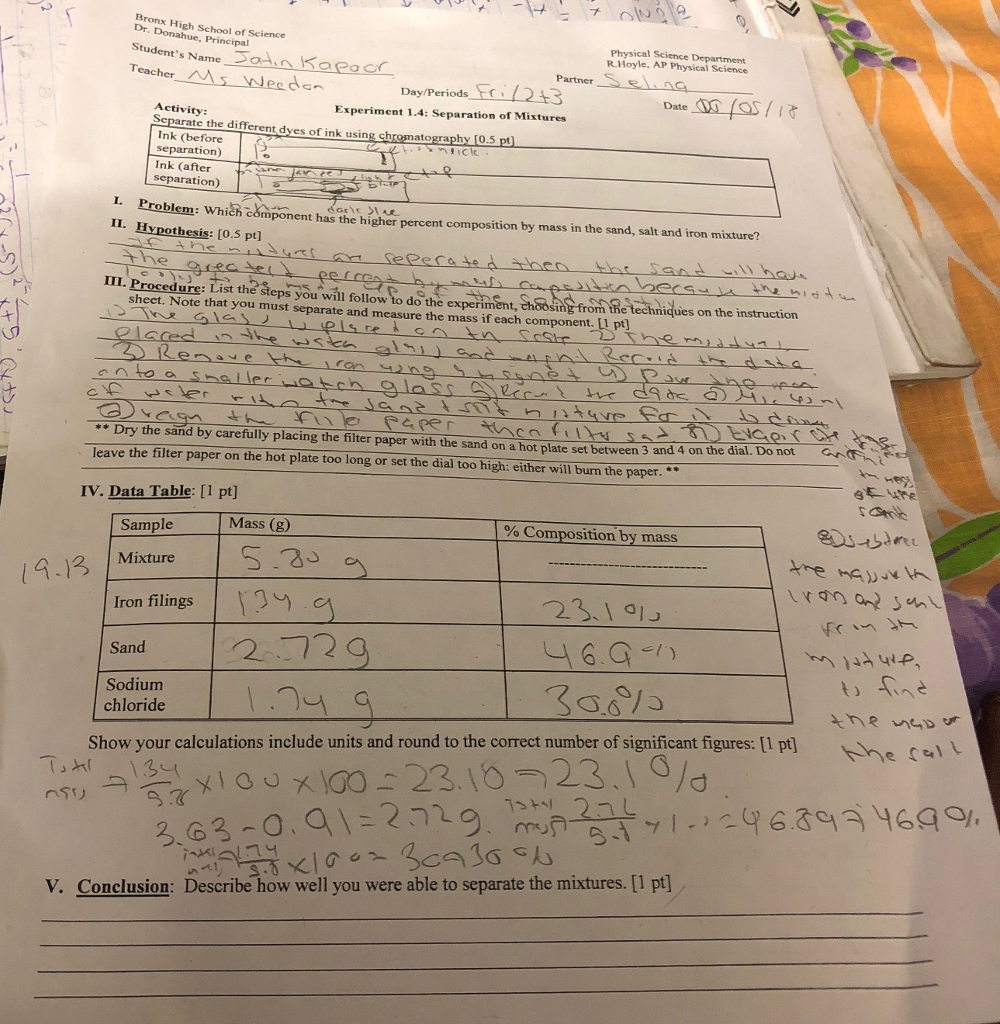
Read the lab thoroughly. Answer the pre-lab questions that appear at the end of this lab exercise. Purpose A heterogeneous mixture of iron Fe salt NaCl and sand SiO 2 will be separated by physical means and the percent composition of each will be determined.
In physical properties when separating them by specific materials for the mixture s such as stirring rod. Furthermore chemicals such as sand sodium chloride NaCl amm onium chloride. A mixture may be a solid liquid gas or some combination of those states.
Mixtures can be found almost every wher in our everyday lifes and some common examples are sand and water salt and water sugar and salt Due to the fact that mixture are not chemically combined They can easily be separated into component substances by using physical means. Separation of a Mixtures Lab Report. 3 pages 1075 words.
The purpose of the experiment was to separate an initial heterogeneous mixture composed of 500 grams of salt 200 grams of sand 500 mL of water 1500 grams of pebbles and 100 gram of iron filings and leave as much salt as possible remaining. Separation of a Mixture Lab Report Abstract The purpose of the experiment was to separate an initial heterogeneous mixture composed of 500 grams of salt 200 grams of sand 500 mL of water 1500 grams of pebbles and 100 gram of iron filings and. Separation Techniques If one component of the mixture has magnetic properties you can use a magnet to separate the mixture.
Cobalt iron and nickel all have magnetic properties. Note that not all metals are magnetic and magnetism is not a method to separate metals from non-metals. Gold silver and mercury are examples of non-metal substances.
Separation of a Mixture of Solids Lab Report - Separation. Fix a separating funnel in a stand. Pour about 50ml of a mixture of oil and water through a filter funnel into a separating funnel.
Close the separating funnel using a lid. Take the funnel from the stand and invert it. Now shake the funnel gently and slowly.
Separation of the Components of a Mixture Pre-lab Questions Before beginning this experiment in the laboratory you should be able to answer the following questions. Classify each of the following as a pure substance or a mixture. If it is a mixture state whether it is heterogeneous or homogeneous.
A concrete b tomato juice c marble d. Separation of Mixtures of Solids - Lab Report Example. Separation of Mixtures of Solids paper contains an experiment that was performed to become familiar with the separation of mixtures containing solid components.
The total weight of the mixtures components after separation was less than the initial weight of the mixture. Chem 101 Lab Report 2. Mixtures are made up of substances or components.
If the mixture is fairly uniform in composition properties and its overall appearance it is homogenous. If the component parts are clearly separated it is heterogeneous. In order to identify the components in a mixture methods must be used to sort out the components.
What physical properties did you use to get the MMs out of the mix. Color shape size texture. Explain that sometimes the need to separate a mixture can be life and death- examples include separating pure water from contaminants or separating blood components for use in medicine.
Play the video Blood Component Processing linked here and. The result is a mixture in which each component retains its individual identity and properties. The separation of the components of a mixture is a problem frequently encountered in chemistry.
The basis of the separation is the fact that each component has a different set of physical and chemical properties. The components are pure substances. Is a separation technique used to separate the components of a mixture containing an undissolved solid in a liquid by using a funnel lined with filter paper to.
The percent composition of any component in an unknown mixture is determined by multiplying the. Most mixtures can be separated by physical change. Because a mixture is a physical combination of.
Not Remove the mixture from the hotplate once is begins to boil. Separation of a Mixture Lab. The purpose of this lab is for you to apply your new knowledge of mixtures and separation techniques and to apply the scientific method to a problem.
The problem is that you have a heterogeneous mixture of different compounds and you have to develop a method for separating the mixture into its components. Start studying Experiment 2. Separation of the Components of a Mixture.
Learn vocabulary terms and more with flashcards games and other study tools. Lab Report on Solubility 2677 Words 11 Pages. Separation of the Components of a Mixture General Chemistry 1 Chem 101 ISP SCUHS Report 2 January 26 2014 Abstract The analyses of mixture were to distinguish and identify homogeneous mixture by using the techniques of decantation and sublimation.