OA chemical reaction does not occur for this question. The reaction with water.

Mg2 sodium Express your answer as an ion.
Express your answer as an ion. Ions are expressed by the Chemical Symbol of the element s followed by a superscript of the charge the ion carries. For example an Oxide ion would be Oxide is an oxygen anion which gains 2. Chemistry questions and answers.
Mg Express your answer as an ion. N Express your answer as an ion. F Express your answer as an ion.
I Express your answer as an ion. Mg Express your answer as an ion. N Express your answer as an ion.
Express your answer as a balanced net ionic equation. Identify all of the phases in your answer. Part B Identify the spectator ion or ions in this reaction.
Express your answer as an ion. If there is more than one answer separate them by a comma. Chemistry Reactions in Solution Reactions Between Ions in Solutions.
Express your answer as an ion. Mg2 sodium Express your answer as an ion. Selenium Express your answer as an ion.
Se2-Al2SO43 an antiperspirant Spell out the full name of the compound. CaCO3 an antacid Spell out the full name of the compound. Write the ions present in a solution of eqNa_2CO_3 eq.
Express your answers as chemical formulas separated by a comma. What is the concentration of the unknown eqH_3PO_4 eq solution. Express your answer as a chemical formula or an ion.
Nothing Request Answer Part B Write the name of the conjugate base found in part A. Spell out the full name of the compound or ion. Request Answer Part C Write the formula of the conjugate base for acid H2O.
Express your answer as a chemical formula or an ion. Nothing Request Answer Part D. Express your answer as a chemical symbol or as an ion.
Write the number of electrons that were lostgained to form Ar. Express your answer as a signed integer. For example if electrons are lost use minus sign.
0 electron s Write the ion atom that has 16 protons and has gained 2 electrons. Express your answer as a chemical formula or an ion. Write the name of the conjugate base found in part A.
Spell out the full name of the compound or ion. Write the formula of the conjugate base for acid H2O. Express your answer as a chemical formula or an ion.
Write the name of the conjugate base found in part C. SubmitRequest Answer Par OneClass. Predict the ion formed by each element.
Part A Rb Express your answer as an ion. LIMITED TIME OFFER. GET 20 OFF GRADE YEARLY SUBSCRIPTION.
Furthermore What is the conjugate base of HSO4 HSO4 Express your answer as a chemical formula Therefore the sulfate ion SO24 is the conjugate base of HSO4. Finally What is the conjugate base of HSO4 ion When an acid dissociates into its ions in water it loses a hydrogen ion. State the number of electrons lost or gained when the following elements form ions.
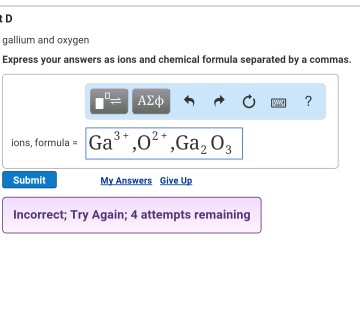
Sr Express your answer as an integer. Express your answer as an isotope. Atomic mass 6972 amu Gallium has two naturally occurring isotopes.
Ga-69 with mass 689256 amu and a natural abundance of 6011 and Ga-71 with mass 709247 amu and a natural abundance of 3989. Express your answer as an ion. Write the formula for the polyatomic ion in FeHCO33.
Express your answer as an ion. Write the name for FeHCO33. Spell out the full name of the compound.
Express your answer as a chemical formula. Express your answer as an ion. Express your answer as an ion.
Write a formula for the compound that forms between calcium and each polyatomic ion. Part A hydroxide Express your answer as a chemical formula. Part B chromate Express your answer as a chemical formula.
Part C phosphate Express your answer as a chemical formula. Part D cyanide Express your answer as a chemical formula. What is the mass number of an ion with 108 electrons 158 neutrons and a 1 charge Express your answer as an integer.
The ions has 1 charge and contains 108 electrons it means the ion has losses one electron the number of proton must be 109. This is an oxide ion with a negative two charge. With eight protons we are necessarily looking at oxygen but with 10 electrons the net charge on the ion is minus two.
This charge is written as a superscript in the upper right corner relative to the symbol for oxygen atoms Closest I can get to the write the symbol in this answer is O2- the - sign should be part of the superscript. Part A Be ion Express your answer as a signed integer in the form 3. Request Answer Previous Answers Submit Incorrect.
3 attempts remaining Part B Sc ion Express your answer as a signed integer in the form 3. Express your answer in condensed form in order of increasing orbital energy. For example He2s22p2 would be entered as He2s22p2.
Part B What is the ground-state electron configuration of the oxide ion O2. Express your answer in condensed form in. Ca Express your answer as an ion.
ΑΣφ NA chemical reaction does not occur for this question. Part B Express your answer as an ion. OA chemical reaction does not occur for this question.
Express your answer numerically as a decimal or improper fraction. The bond order for a molecule is the number of bonds between a pair of atoms. For example for the oxygen molecule O O the bond order is two.
For the hydrogen molecule H - H the bond order is one. Bond order indicates the. Enter the formula for the polyatomic ion in Cu2SO3.
Express your answer as an ion. - For All Answers. October 27 2021 by sarah yalton.
Enter the formula for the polyatomic ion in Cu2SO3. Express your answer as an ion. Enter the formula for the polyatomic ion in Cu2SO3.
Express your answer as an ion. Write the formula for the polyatomic ion of ba3po42. Express your answer as an ion.
Express single charges as not 1 - 8998087. Express your answer as an ion. Want to see the step-by-step answer.
Check out a sample QA here. Want to see this answer and more. Experts are waiting 247 to provide step-by-step solutions in as fast as 30 minutes See Answer.
You should ask What is the conjugate base of HS04-. The answer is S042. The reaction with water.
HSO4- H2O SO4- H3O HSO4- is acid SO4- is conjugate base. H20 is base H30 is conjugate acid. It is not possible to put this ion HSO4- into solution as it is.
It has to accompany something like sodium hydrogen sulfate or calcium sulfate.