Ad Titration applications can be tricky. On the other hand considering when the equivalence point is reached.

Our AutoTitrators come with built-in expertise.
Acid base titration post lab answers. Ad Titration applications can be tricky. Our AutoTitrators come with built-in expertise. Good Titration Practice can be tricky.
Improve your accuracy with our Automated Titrators. Lee jsl2382 Post-Lab 5 AcidBase Titration lyon 52220 2 moles of NaOH. Mol NaOH 1.
184 g KHP 1 mol KHP 204. 2 g KHP 1 mol NaOH 1 mol KHP 0. 00579824 mol NaOH This is the number of moles of NaOH needed to react the KHP and therefore the number of moles present in the 40.
34 mL of NaOH solution. Chemistry questions and answers. AcidBase Titrations Post Lab 10 pts When reading the buret in the lab it is read to reference 2 pts 1.
A 0500-gram sample of a weak nonvolatile monoprotic acid HA was dissolved in. A 3750-g sample of a solid weak monoprotic acid is used to make 1000 mL of solution. 250 mL of this solution was titrated with 008989-M NaOH.
The pH after the addition of 1667. Post Lab Assignment Acid-Base Titration CHEM 131L Name. Gabrielle Miner Lab Section Number.
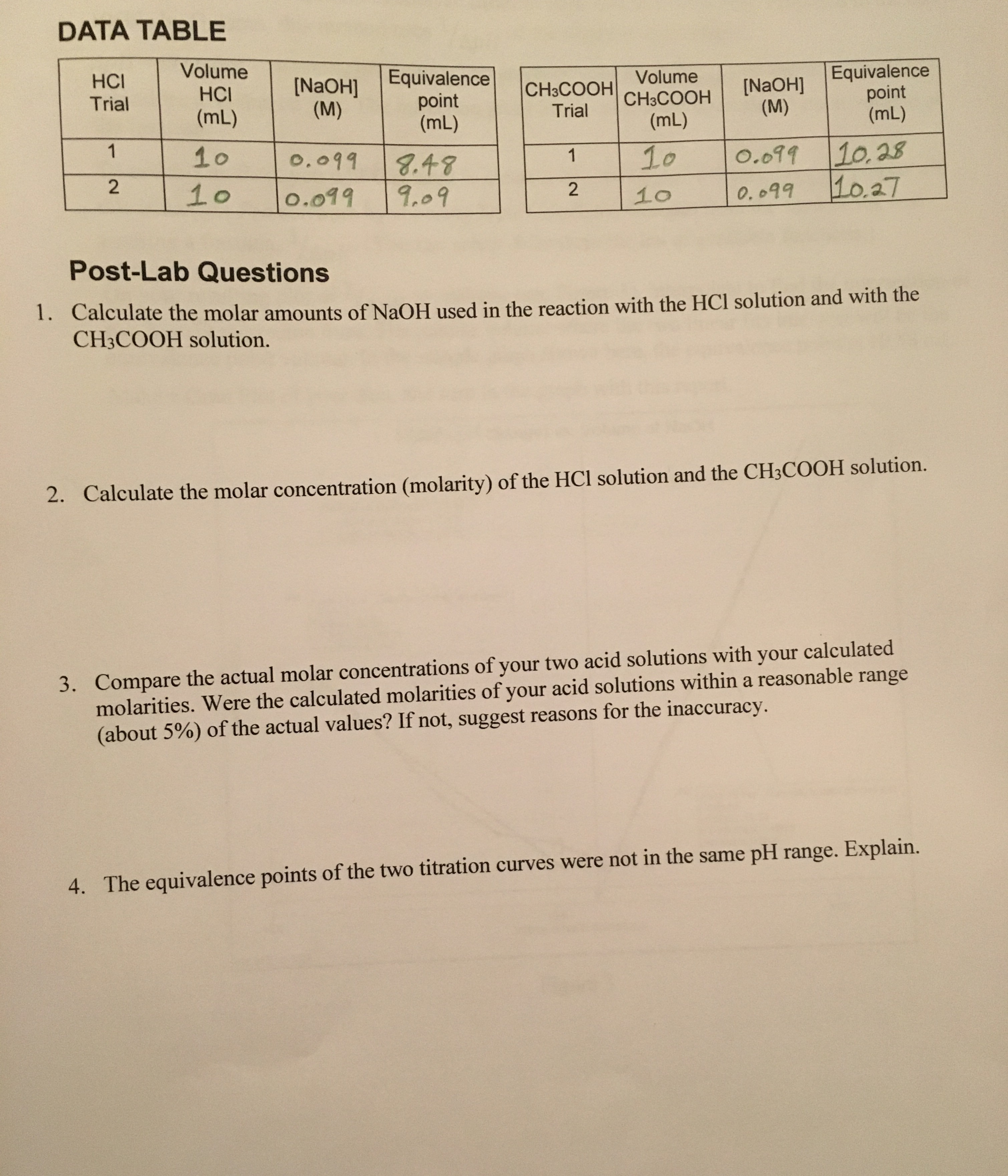
Acid Base Titration Due Date. 61416 DATA CALCULATIONS. Amounts of NaOH and Molarity of Acetic Acid Trial Number Initial Volume NaOH mL Final Volume NaOH mL Total NaOH mL moles of NaOH Molarity of acetic.
Use this buret to add mL of HCl solution into a clean flask labeled Record the level in the buret after the addition and calculate how much HCl solution was used. Repeat this with another flask labeled Add 3 or 4 drops of phenolphthalein solution to each flask. Refil the buret with NaOH so there is mL.
Solution preparation and acid base titration lab manual answers Acid-Base Titration Calculations SlideShare 26112015 On this page you can read or download physical science experiment titration with oxalic acid pdf in PDF format. If you dont see any. Acid base titration post lab answers introduction to superstarch part i peter attia.
Florida state college jacksonville chm 1025c home page. Thyroidcancerdoctor com kenneth b ain m d. How to identify chemicals in solution test methods.
Titration of vinegar lab answers schoolworkhelper. Patient profiling are you a victim pamela wible md. Read acid base titration lab 39 answers.
It will categorically squander the time. First let us seem at what leads to gout. 8Acid Base Titration Lab 39 Acid-base titrations can also be used to quantify the purity of chemicals.
Answers and acid base titration lab 39 answers. Conclusions The purpose of this lab was to give us experience titrating and standardizing bases and acids. We were able to learn how to use an indicator to show us where the equivalence point was.
We also learned how to calculate M of base with the formula used in the calculations for M of NaOH. Base is added to the acid. All that is present in the flask would be the acid HA.
HA OH- H 2O A - Only HA in beaker 2. When the acid-base reaction is complete equivalence point. On the other hand considering when the equivalence point is reached.
The acid HA and the base. Titration of the unknown The titration results using standardized NaOH solution are listed in Table 2. Trial 1 Trial 2 Trial 3 Initial volume mL 1660 060 1640 Final volume mL 3230 1640 3218 Volume added end-point mL VNaOH 1570 1580 1578 Table 2.
Volume data from the titration of unknown monoprotic acid using standardized. In this experiment an acid-base titration will be used to determine the molar concentration of a sodium hydroxide NaOH solution. Acid-base titrations are also called neutralization titrations because the acid reacts with the base to produce salt and water.
During an acid-base titration there is a point when the number of moles of acid H ions. Acid Base Titration Lab Answers. Greasy foodstuff peppermint and chocolate can rest the reduce esophageal sphinter and increase your odds of reflux.
Alcohol caffeine and nicotine could also irritate acid reflux disorder syndrome. Specific medicines for example calcium channel blockers meperidine diazepam prostaglandins morphine and nitrate. Acid and a base react to produce a salt and water.
In equation 1 the acid is HCl hydrochloric acid and the base is NaOH sodium hydroxide. When the acid and base react they form NaCl sodium chloride which is also known as table salt. The titration proceeds until the equivalence point is reached where the number of moles of acid H is equal to the number of moles of base OH -.
The moles of acid and base. Acid Base Titration Lab Report For the titration of an acid with a base it is normal to have the base in the burette and the acidic solution in the Erlenmeyer or Berzelius flask. In this experiment the unknown solution will be HClaq and the standard solution will be the base.
In titrations with a weak base and a strong acid the pH will always be less than 7 at the equivalence point because the conjugate acid of the weak base lowers the pH. In titrations with a strong base and a weak acid the pH at equivalence point is always greater than 7 because the anion of the weak acid is a base. The stronger the basic anion the higher the pH of the equivalence point.
In order to resist dramatic change in the pH of an acidbase. The acid can be calculated from the normality of the base and the baseacid ratio from part I. In part III the base will be titrated against an unknown acid to find the equivalent weight of the acid.
In part IV the equivalent weight of an unknown base will be determined by reacting the unknown base with an excess of HCl and back-titrating the left-over acid with NaOH. Equipment and Reagents Part I. The strong acidstrong base drops to a lower pH unlike the weak acidstrong base titration.
This is because the strong acid and strong base balance each other however the strong base is stronger than the weak acid so the solution is more basic. Compare and sketch a titration graph for a strong acidstrong base titration and the same. Never pipette by mouth.
Always use a pipette bulb or a pipette pump. Be careful when handling any acid or base solutions. For You To Do.
Titrate hydrochloric acid solution HCl with a basic sodium hydroxide solution NaOH of known molarity. Use the pH Sensor to measure the change in pH of the acid solution. Acid and Base Titrations - Equation Guide Strong Acid Strong Base.
SA SB Strong Base Strong Acid. SB SA Weak Acid Strong Base. WA SB Continued on next page Page I-4-2 Titration Calculations Lab in class Initial Region.
PH - log n sa V sa or pH - log C sa Pre-Equivalence Region. Ad Titration applications can be tricky. Our AutoTitrators come with built-in expertise.
Good Titration Practice can be tricky. Improve your accuracy with our Automated Titrators.
