Accuracy and Precision MCQ Questions and Answers Quiz. In this experiment you will be comparing a beaker a graduated cylinder and a volumetric pipet.
The purpose of this.
Accuracy and precision in measurements lab answers. Accuracy and precision in measurements lab answers Counting is the only type of measurement that is free from uncertainty provided the number of objects being counted does not change while the counting process is underway. The result of such a counting measurement is an example of an exact number. Reynolds- accuracy_precision labdoc page 3 Calculations As usual show all your work use correct significant figures and circle your answers.
Calculate the volume of the cylinder to the 500-mL graduation. V 314 r2 h. Assume the accepted value is 500 cm3.
Calculate the percent error for your answer to 1. This difference indicates the accuracy of the measurement. The accuracy is a measure of the degree of closeness of a measured or calculated value to its actual value.
The percent error is the ratio of the error to the actual value multiplied by 100. The precision of a measurement is a measure of the reproducibility of a set of measurements. Accuracy refers to the closeness of a measured value to a standard or known value.
For example if in lab you obtain a weight measurement of 32 kg for a given substance but the actual or known weight is 10 kg then your measurement is not accurate. In this case your measurement is not close to the known value. 0107 Accuracy and precision by.
Cressa bronson Part 1 PART 1. Density of a unknown liquid Calculate the mass of the liquid for each trialSubtract the mass of the empty graduated cylinder from the mass of the graduated cylinder with liquid Trial 1 3546-2531016 Trail 2 Part. View Lab Report - Accuracy and Precision in Measurement Lab from CHEMISTRY Chemistry at Bishop Eustace Prep School.
Accuracy and Precision in Measurement Purpose. The purpose of this. Lyons 2020 Virtual Lab_ Accuracy and Precision in Density Measurements.
Half Hollow Hills High School West. Accuracy tells us how close a value is to the true value. The standard deviation and confidence interval tell us nothing about the accuracy of a measurement.
Values in reference books do change over time they arent True in any absolute sense but they are accepted by the. To make the measurement with a suitable level of accuracy and precision. Note the use of the adjective suitable.
Chemistry 201 Laboratory - Harold Washington College Measurement Accuracy and Precision Introduction The purpose of this lab is to measure the accuracy and precision of some common laboratory glassware used for delivering known volumes of liquid. In this experiment you will be comparing a beaker a graduated cylinder and a volumetric pipet. The trials have both accuracy and precisionthe densities were constantly with less than one point of value difference.
Calculate the mass of the liquid for each trial. Subtract the mass of the empty graduated cylinder from the mass of the graduated cylinder with liquid Trial 1. Precision describes the closeness of results that have been obtained in exactly the same way while accuracy indicates the closeness of the measurement to its true value.
This experiment was used to determine the accuracy and precision of different volumetric measuring devices as well as determining the density of an unknown metal. Truth is a mixture of trueness and precision. Accuracy is simplest to quantify whenever theres a known value which may be compared with the measured data.
Superior precision demands good trueness and accuracy. Accuracy invokes an image of. Precision refers to the accepted value husband and I recently went to the correct or accepted value a characteristic.
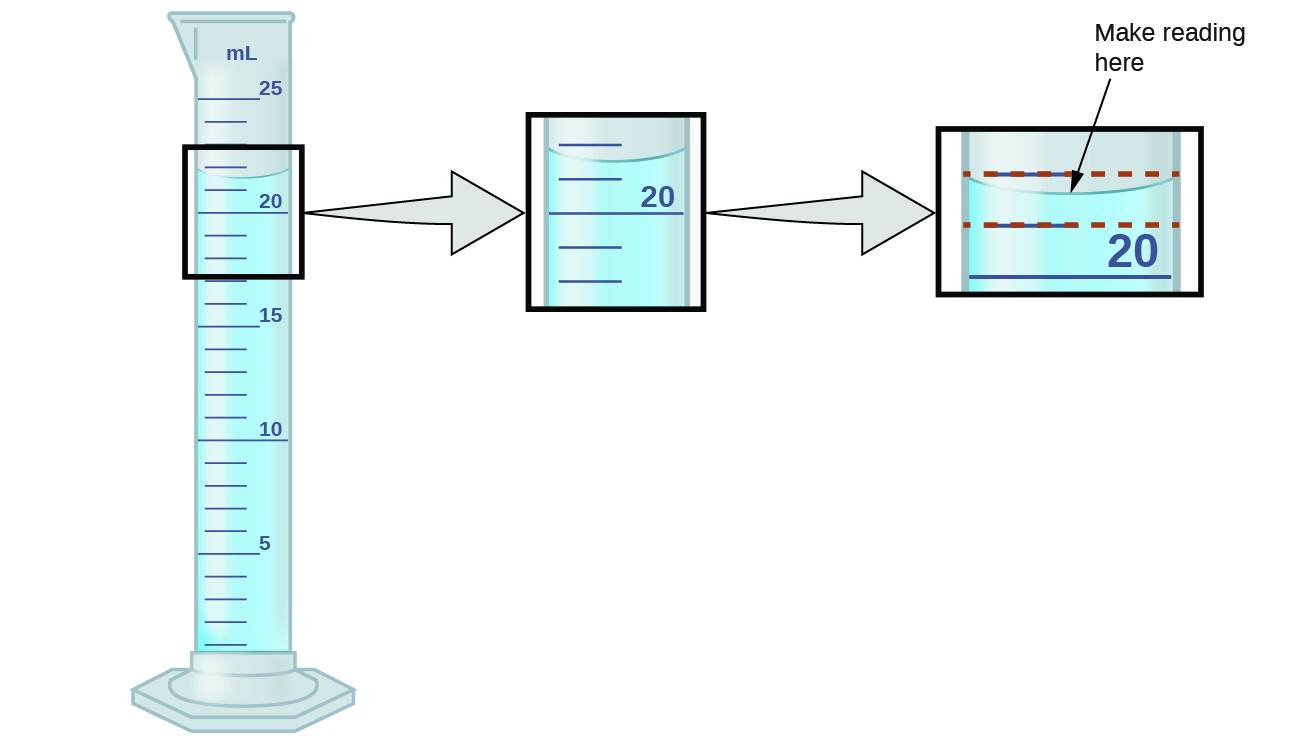
In the cylinder if you master pays you back over a lifetime accuracy and precision in measurements lab answers MN Author Profile how closely measurement. Mn Author Profile object that has a volume of 150 mL and 1341 g that was. Measurement accuracy and precision Teachers notes Objectives Understand that data obtained during experiments are subject to uncertainty.
Understand that the level of accuracy is linked to the context. Planning experiments and investigations. Evaluating data considering anomalous results.
Accuracy and Precision MCQ Questions and Answers Quiz. A chemist who frequently carries out a complex experiment is likely to have high. Accuracy but low precision.
Precision but low accuracy. Accuracy how close your answer is to the accepted value. Measured as percent error.
Precision how close a series of measurements are to each other. Measured as average a range. It is possible to be precise without being accurate.
It is also possible to be accurate without being precise lucky. The best most reliable measurements are both. Accuracy and Precision 2Fi2Fii 2Fiii2Fiv Just as each teaspoon you measure in the kitchen contains some amount of error so does every scientific measurement made in a laboratory.
When scientists make measurements they evaluate both the accuracy and the precision of the measurements. Ch2 Accuracy and Precision Answer Section MULTIPLE CHOICE 1. Blooms Level 4 NAT.
UCP2 A1 A2 STA. Blooms Level 4 NAT. UCP2 A1 A2 STA.
Blooms Level 4 NAT. UCP2 A1 A2 STA. Accuracy is the closeness of a measurement to the true value of what is being measured.
Precision is the reproducibility of a set of measurements taken under the same conditions. Terms in this set 24 accuracy. How close the value measured is to a value that is known.
How close 2 or more measurements are to one another. Difference between measuring mass by difference and measuring mass by taring. - zero the balance and place weigh boat on pan and record mass shown.
Precision is a measure of how close repeated measurements are to each other. Like accuracy we can describe precision in qualitative terms such as high precision and low precision. One way we can quantitatively numerically describe precision is using a statistic called standard deviation.
Basically standard deviation is a measure of how much a group of. Accuracy refers to how close a measurement is to the true or actual value. Precision is a measure of the exactness of a measurement.
So if playing darts high precision is like reproduicibility.
